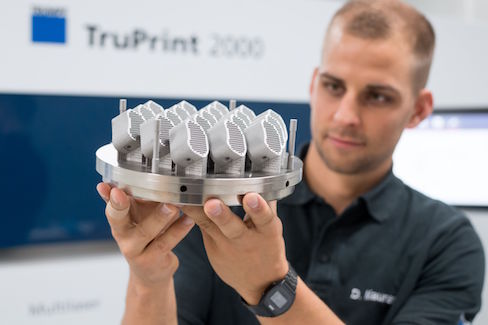
Economical 3D Printer for Medical and Dental Devices
Featuring two 300-watt lasers, melt pool and process monitoring, and end-to-end documentation that corroborates quality, Trumpf’s TruPrint 2000 requires fewer add-ons, making the entry-level investment lower for companies that want to get into additive manufacturing.
Posted: December 3, 2020
Like the TruPrint 1000, the 55-micron-diameter laser beam of Trumpf Inc.’s (Farmington, CT) TruPrint 2000 produces components with smoother surfaces, enhanced quality, and intricate grid structures from titanium, cobalt-chromium alloys, and other materials commonly used to make dental and medical devices. Offering a build volume of 7.9 inches (200 mm) in diameter and 7.9 inches (200 mm) high, the 3D printer’s two 300-watt fiber lasers is highly productive.
The production process is a closed powder circuit under shielding gas. This allows for easy and practical handling, with the highest operator safety.
The machine processes powder in an inert gas environment, which prevents contaminants from infiltrating the powder circuit – a major advantage when manufacturing sensitive medical devices. The shielding gas flows back to front, which enhances part quality.
Built-in melt pool monitoring and comprehensive process monitoring enable operators to maintain consistent quality. In the event of an error, the system notifies the operator.
The operator can remove excess powder from the component within the machine rather than having to take it out and unpack it at a separate station. This is easier and saves time when dealing with the smaller build chambers of 3D printers.
End-to-end documentation corroborates the quality of the printing process, a key prerequisite for the additive manufacturing of medical devices.